Research topics
Multifunctional polymer nanoparticles for imaging and cell/molecular targeting
The medical application of nanotechnology, usually termed nanomedicine, has given a crucial impulse to the development of various types of drug-loaded nanocarriers, such as liposomes, nanoparticles, micelles, etc. In our group, a great deal of effort is focused on the engineering of biodegradable polymer nanoparticles able to serve as efficient diagnostic and/or therapeutic tools against severe diseases, such as cancer, infectious or neurodegenerative disorders.
Poly(alkyl cyanoacrylate) nanoparticles
Poly(alkyl cyanoacrylate) (PACA) nanoparticles have opened exciting perspectives for drug delivery due to their biodegradability and their non-toxicity. For instance, doxorubicine-loaded poly(isobutyl cyanoacrylate) nanoparticles have reached phase III clinical trials against primary liver cancer (LIVATAG®). However, the design of functionalized PACA nanoparticles for imaging and/or targeting is still very challenging due to the high reactivity of alkyl cyanoacrylate monomers [WIREs Nanomed. Nanobiotechnol. 2009]. The suceptibility to hydrolysis of PACA polymers also prevents efficient post-functionalization approaches.
Fluorescent PACA nanoparticles. 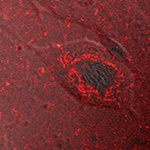 In order to circumvent the drawbacks usually
encountered with the use of small fluorescent dyes encapsulated
into nanoparticles (e.g., leakage), rhodamine B-tagged poly(alkyl cyanoacrylate) amphiphilic copolymer nanoparticles have been prepared by tandem Knoevenagel condensation-Michael addition from the corresponding cyanoacetates derivatives [Chem. Commun. 2010]. They have been used for human brain endothelial cell imaging, allowing their uptake and intracellular trafficking (see figure) to be finely observed. We also developped PEGylated PACA nanoparticles loaded with visible- and near-infrared-emitting quantum-dots (QDs) with high encapsulation yields, suitable for in vitro and in vivo imaging, respectively [Soft Matter 2011].
In order to circumvent the drawbacks usually
encountered with the use of small fluorescent dyes encapsulated
into nanoparticles (e.g., leakage), rhodamine B-tagged poly(alkyl cyanoacrylate) amphiphilic copolymer nanoparticles have been prepared by tandem Knoevenagel condensation-Michael addition from the corresponding cyanoacetates derivatives [Chem. Commun. 2010]. They have been used for human brain endothelial cell imaging, allowing their uptake and intracellular trafficking (see figure) to be finely observed. We also developped PEGylated PACA nanoparticles loaded with visible- and near-infrared-emitting quantum-dots (QDs) with high encapsulation yields, suitable for in vitro and in vivo imaging, respectively [Soft Matter 2011].
Interaction with the amyloid-β 1-42 peptide. 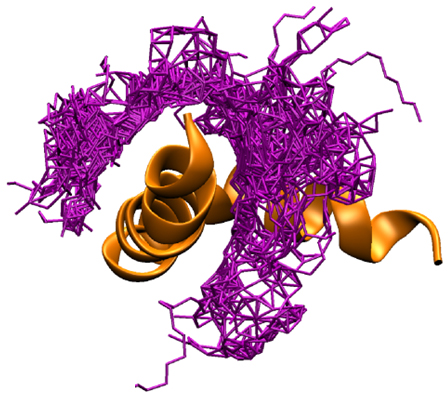 To detect interaction between functionalized nanoparticles and the amyloid-β 1-42 peptide (Aβ1-42), an important biomarker of the Alzheimer's disease (AD), we developped a reliable analytical tool based on capillary electrophoresis with laser-induced fluorescence detection (CE-LIF) [Anal. Chem. 2010]. Unexpectedly, we discovered that the PEG corona of long-circulating PACA
nanoparticles favors interaction with Aβ1-42 both in solution and in serum [ACS Nano 2012a]. In silico and modeling experiments highlighted the mode of PEG interaction with the Aβ1-42 peptide and its conformational changes at the nanoparticle surface (see figure). These nanoparticles might act as a
peptide sequester in the bloodstream, carrying the
peptide to the liver where it could be enzymatically
cleaved and degraded.
To detect interaction between functionalized nanoparticles and the amyloid-β 1-42 peptide (Aβ1-42), an important biomarker of the Alzheimer's disease (AD), we developped a reliable analytical tool based on capillary electrophoresis with laser-induced fluorescence detection (CE-LIF) [Anal. Chem. 2010]. Unexpectedly, we discovered that the PEG corona of long-circulating PACA
nanoparticles favors interaction with Aβ1-42 both in solution and in serum [ACS Nano 2012a]. In silico and modeling experiments highlighted the mode of PEG interaction with the Aβ1-42 peptide and its conformational changes at the nanoparticle surface (see figure). These nanoparticles might act as a
peptide sequester in the bloodstream, carrying the
peptide to the liver where it could be enzymatically
cleaved and degraded.
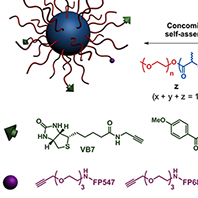
Targeted PACA nanoparticles.  We also designed a versatile and ligand-functionalized PACA nanoparticulate platform by 'click' chemistry [Macromolecules 2008], gathering all together crucial
features required for active targeting and drug delivery (i.e., biodegradable, PEGylated, fluorescent and targeted). The multifunctional nanoparticles were successfully used to target two major pathologies;
namely cancer and Alzheimer's disease, via their functionalization by appropriate biologically active ligands [ACS Nano 2012b]. Against cancer cells, we used biotin to target biotin receptor that are overexpressed at the surface of many cancer cells (see figure), whereras Aβ1-42 peptide was targeted by means of curcuminoid derivatives and an anti-Aβ1-42 peptide antibody. The antibody-decorated nanoparticles exhibited higher affinity toward
Aβ1-42 species comparatively to other kinds of colloidal systems and led to significant
aggregation inhibition and toxicity rescue of Aβ1-42 at low molar ratios.
We also designed a versatile and ligand-functionalized PACA nanoparticulate platform by 'click' chemistry [Macromolecules 2008], gathering all together crucial
features required for active targeting and drug delivery (i.e., biodegradable, PEGylated, fluorescent and targeted). The multifunctional nanoparticles were successfully used to target two major pathologies;
namely cancer and Alzheimer's disease, via their functionalization by appropriate biologically active ligands [ACS Nano 2012b]. Against cancer cells, we used biotin to target biotin receptor that are overexpressed at the surface of many cancer cells (see figure), whereras Aβ1-42 peptide was targeted by means of curcuminoid derivatives and an anti-Aβ1-42 peptide antibody. The antibody-decorated nanoparticles exhibited higher affinity toward
Aβ1-42 species comparatively to other kinds of colloidal systems and led to significant
aggregation inhibition and toxicity rescue of Aβ1-42 at low molar ratios.
Polyester nanoparticles
 Polyesters are biodegradable FDA-approved polymers for use in Humans and have been widely used to prepare nanocarriers for drug delivery applications. However, the accurate functionalization of polyester nanoparticles to achieve active targeting is not straightforward and generally not orthogonal, which could result in multisite attachment [Chem. Soc. Rev. 2013].
Polyesters are biodegradable FDA-approved polymers for use in Humans and have been widely used to prepare nanocarriers for drug delivery applications. However, the accurate functionalization of polyester nanoparticles to achieve active targeting is not straightforward and generally not orthogonal, which could result in multisite attachment [Chem. Soc. Rev. 2013].
In collaboration with Sanofi, we have been interested in applying 'click' chemistry to long-circulating poly(lactic acid)-block-poly(ethylene glycol) (PLA-b-PEG) copolymer nanoparticles (see figure) [WO 2013127949]. This strategy has been illustrated by the preparation of a large library of different nanoparticles, such as, ligand-decorated nanoparticles (with biotin, folic acid or anisamide), fluorescent nanoparticles (UV-Vis or near-infrared dyes), and multifunctional nanoparticles decorated with a targeting ligand and a fluorescent probe [Chem. Mater. 2014]. Optimal ligand availability was determined by surface plasmon resonance and successful targeting was demonstrated by in vitro experiments on different cancer cell lines.
Controlled radical polymerization: mechanism and synthesis of biomaterials
Controlled radical polymerization (CRP) techniques, such as nitroxide-mediated polymerization (NMP), atom-transfer radical polymerization (ATRP) and reversible addition-fragmentation chain transfer (RAFT) polymerization, enable the synthesis of well-defined, complex and functional macromolecular architectures. We are excited to explore new areas of CRP techniques and to take the most of them to design innovative structures and novel biomaterials.
Nitroxide-mediated polymerization
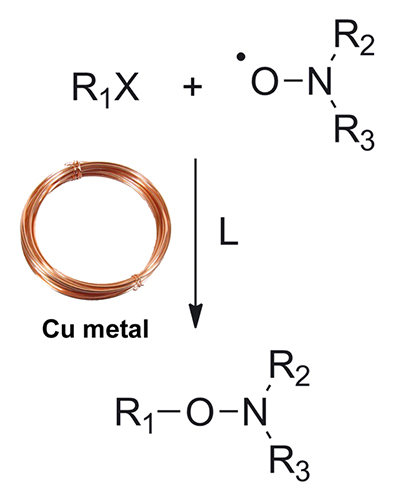 Our group has a strong expertise in CRP techniques and especially in nitroxide-mediated polymerization (NMP) [Prog. Polym. Sci. 2013]. Among our different achievements in this field, we proposed an simple yet efficient method for the synthesis of alkoxyamine initiators mediated by copper metal [Polym. Chem. 2010] and reported optimized conditions for the successul NMP of isoprene [Macromolecules 2011]. We are also very interested by the NMP of methacrylic esters through the copolymerization approach. It consists in adding a very small amount of a comonomer such as styrene (typically 4-9 mol.%) during the NMP of methacrylic esters to obtain all the features of a controlled/living system [Macromolecules 2009]. We have extended this concept to other monomers such as oligo(ethylene glycol) methacrylate [Macromolecules 2008] to prepare PEG-based materials and also discovered that acrylonitrile can be used an efficient 'controlling' comonomer [JPSA 2010].
Our group has a strong expertise in CRP techniques and especially in nitroxide-mediated polymerization (NMP) [Prog. Polym. Sci. 2013]. Among our different achievements in this field, we proposed an simple yet efficient method for the synthesis of alkoxyamine initiators mediated by copper metal [Polym. Chem. 2010] and reported optimized conditions for the successul NMP of isoprene [Macromolecules 2011]. We are also very interested by the NMP of methacrylic esters through the copolymerization approach. It consists in adding a very small amount of a comonomer such as styrene (typically 4-9 mol.%) during the NMP of methacrylic esters to obtain all the features of a controlled/living system [Macromolecules 2009]. We have extended this concept to other monomers such as oligo(ethylene glycol) methacrylate [Macromolecules 2008] to prepare PEG-based materials and also discovered that acrylonitrile can be used an efficient 'controlling' comonomer [JPSA 2010].
Controlled radical ring-opening polymerization
 The crucial need for more environmentally friendly plastic materials is currently stimulating the development of degradable vinyl polymers [Nature Chem. 2015]. A significant part of our research efforts is also focused on controlled radical ring-opening polymerization (rROP) as a way to produce (bio)degradable vinyl polymers for biomedical applications and sustained development [Chem. Rev. 2017]. In this context, we reported the first synthesis of degradable PEG-based copolymers by NMP [Biomacromolecules 2013] using different cyclic ketene acetals (CKA) as precursors of main-chain labile moieties. We have shown that the structure of the CKA is a crucial parameter to reach high monomer conversion, good control of the polymerization and adjustable insertion of CKA in the copolymer. This resulted in tunable degradation of the copolymer, up to complete degradation for a sufficient amount of inserted CKA. The copolymers were show to be not cytotoxic. We also extended this approach to the deisgn of block copolymers with a degradable block [Macromol. Rapid Commun 2014], to the synthesis of dedradable, alternating copolymers based on the RAFT copolymerization between CKA and maleimides [ACS Macro. Letters 2017]. In collaboration with the group of Didier Gigmes, we also developped a new copolymerization system between CKA and vinyl ethers to afford degradable, functional polymers for various applications, including functional polymers, PEGylated nanoparticles, antibacterial surfaces and bioelastomers [Angew. Chem., Int . Ed. 2017].
The crucial need for more environmentally friendly plastic materials is currently stimulating the development of degradable vinyl polymers [Nature Chem. 2015]. A significant part of our research efforts is also focused on controlled radical ring-opening polymerization (rROP) as a way to produce (bio)degradable vinyl polymers for biomedical applications and sustained development [Chem. Rev. 2017]. In this context, we reported the first synthesis of degradable PEG-based copolymers by NMP [Biomacromolecules 2013] using different cyclic ketene acetals (CKA) as precursors of main-chain labile moieties. We have shown that the structure of the CKA is a crucial parameter to reach high monomer conversion, good control of the polymerization and adjustable insertion of CKA in the copolymer. This resulted in tunable degradation of the copolymer, up to complete degradation for a sufficient amount of inserted CKA. The copolymers were show to be not cytotoxic. We also extended this approach to the deisgn of block copolymers with a degradable block [Macromol. Rapid Commun 2014], to the synthesis of dedradable, alternating copolymers based on the RAFT copolymerization between CKA and maleimides [ACS Macro. Letters 2017]. In collaboration with the group of Didier Gigmes, we also developped a new copolymerization system between CKA and vinyl ethers to afford degradable, functional polymers for various applications, including functional polymers, PEGylated nanoparticles, antibacterial surfaces and bioelastomers [Angew. Chem., Int . Ed. 2017].
Polymer-protein/peptides bioconjugates by CRP
 The design of polymer-protein/peptide bioconjugates by CRP techniques is an important area of research [Polym. Chem. 2010]. NMP is perhaps the most adapted technique for biomedical applications due to its simplicity and the absence of toxic reagents. In this view, we reported the synthesis of innocuous PEG-based copolymers in water-rich solutions intended to be used for bioconjugation purposes [Macromolecules 2010]. N-succinimidyl ester-functionalized PEG-based copolymers were then linked to various amine-containing substrates such as a neuroprotective tripeptide and lysozyme [Polym. Chem. 2011]. This represents the first example of peptide/protein PEGylation by the NMP technique. Azlactone-functionalized SG1-based alkoxyamine for NMP and protein bioconjugation has also been developped by our group [Macromolecules 2015].
The design of polymer-protein/peptide bioconjugates by CRP techniques is an important area of research [Polym. Chem. 2010]. NMP is perhaps the most adapted technique for biomedical applications due to its simplicity and the absence of toxic reagents. In this view, we reported the synthesis of innocuous PEG-based copolymers in water-rich solutions intended to be used for bioconjugation purposes [Macromolecules 2010]. N-succinimidyl ester-functionalized PEG-based copolymers were then linked to various amine-containing substrates such as a neuroprotective tripeptide and lysozyme [Polym. Chem. 2011]. This represents the first example of peptide/protein PEGylation by the NMP technique. Azlactone-functionalized SG1-based alkoxyamine for NMP and protein bioconjugation has also been developped by our group [Macromolecules 2015].
Prodrug nanoparticles
Prodrugs are inactive, bioreversible derivatives of active drug molecules that must undergo an enzymatic and/or chemical transformation in vivo to release the active parent drug. Inspired by this concept, we are currently working on the design of innovative polymer prodrug nanoparticles to tackle the common limitations of traditional nanoparticulate systems in which drugs are physically encpasulated (e.g., burst release, poor drug loadings, etc.) [Polym. Chem. 2014].
Squalene-based prodrug nanoparticles
 A major breakthrough in this field has recently been reported by our group and was termed 'squalenoylation'
A major breakthrough in this field has recently been reported by our group and was termed 'squalenoylation'  . It consists in the coupling of squalene (Sq), a natural lipid, to a variery of different drugs to prepare high drug loading squalene-drug nanoparticles by self-assembly in aqueous solution of the corresponding conjugates. Among the different systems, Sq-gemcitabine (Gem) nanoparticles afforded remarkable anticancer activity against many different solid tumors in vivo. In the past few years, we focused our efforts on the PEGylation of Sq-Gem nanoparticles to confer them with long-circulating abilities [Adv. Funct. Mater. 2008]. Also, we developped the first Sq-based multifunctional system (i.e., therapeutic, traceable and targeted) by a simple co-self-assembly of the corresponding building blocks (i.e., Sq-Gem, Sq-dye and Sq-ligand), that demonstrated enhanced uptake by cancer cells [Chem. Commun. 2014].
. It consists in the coupling of squalene (Sq), a natural lipid, to a variery of different drugs to prepare high drug loading squalene-drug nanoparticles by self-assembly in aqueous solution of the corresponding conjugates. Among the different systems, Sq-gemcitabine (Gem) nanoparticles afforded remarkable anticancer activity against many different solid tumors in vivo. In the past few years, we focused our efforts on the PEGylation of Sq-Gem nanoparticles to confer them with long-circulating abilities [Adv. Funct. Mater. 2008]. Also, we developped the first Sq-based multifunctional system (i.e., therapeutic, traceable and targeted) by a simple co-self-assembly of the corresponding building blocks (i.e., Sq-Gem, Sq-dye and Sq-ligand), that demonstrated enhanced uptake by cancer cells [Chem. Commun. 2014].
Polymer prodrug nanoparticles
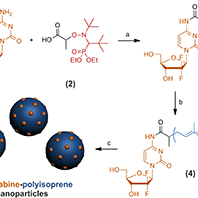
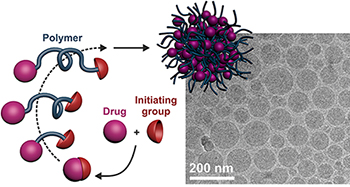 We are also very interested in the design of polymer prodrug nanoparticles as a way to combine both the advantages of the prodrug strategy and the flexibility offered by macromolecular synthesis. We recently developped a new concept termed "drug-initiated" method [Chem. Mater. 2016] which consists in the synthesis of well-defined drug-polymer amphiphiles by CRP of a hydrophobic monomer from an hydrophilic drug [Angew. Chem., Int. Ed. 2013]. The flexibility of this approach was illustrated by the synthesis of different polymer promoeties such as polyisoprene (isoprene being the basic structural motif of many biocompatible natural terpenes) or a naturally occuring lipid-containing polymethacrylate by either NMP or RAFT polymerizations [Biomacromolecules 2013]. Biological evaluations successfully demonstrated both the in vitro and in vivo anticancer activity on solid pancreatic tumors in mice [Chem. Mater. 2014]. This approach was applied to other drugs like cladribine [Chem. Mater. 2016], and to a fluorescent dye [Chem. Commun. 2017] for the preparation of composite nanoparticles by co-nanoprecipitation.
We are also very interested in the design of polymer prodrug nanoparticles as a way to combine both the advantages of the prodrug strategy and the flexibility offered by macromolecular synthesis. We recently developped a new concept termed "drug-initiated" method [Chem. Mater. 2016] which consists in the synthesis of well-defined drug-polymer amphiphiles by CRP of a hydrophobic monomer from an hydrophilic drug [Angew. Chem., Int. Ed. 2013]. The flexibility of this approach was illustrated by the synthesis of different polymer promoeties such as polyisoprene (isoprene being the basic structural motif of many biocompatible natural terpenes) or a naturally occuring lipid-containing polymethacrylate by either NMP or RAFT polymerizations [Biomacromolecules 2013]. Biological evaluations successfully demonstrated both the in vitro and in vivo anticancer activity on solid pancreatic tumors in mice [Chem. Mater. 2014]. This approach was applied to other drugs like cladribine [Chem. Mater. 2016], and to a fluorescent dye [Chem. Commun. 2017] for the preparation of composite nanoparticles by co-nanoprecipitation.